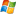The Hoffman electrolysis apparatus provides its users with both convenience, ease of operation, and very precise and exact measurements. The Hoffman electrolysis apparatus is frequently utilized for clinical applications, research purposes, and even for student experiment applications. The Hoffman electrolysis apparatus was invented by August Wilhelm von Hoffman. The most common use of the Hoffman electrolysis apparatus is for studying the electrolysis of water.
The highly accurate and precise Hoffman electrolysis apparatus eliminates any need for guesswork from taking measurements. This is because it is outfitted with two clearly marked gas collecting tubes with a graduated 60 ml capacity. These tubes are clearly marked with divisions of 0.2 ml, so the measurements are exact every time.
Durability and Ease of Use
Both highly durable and simple to use, the Hoffman electrolysis apparatus is among the bet available options for studying the electrolysis of water. In addition including the two telescoping gas tubes, the Hoffman electrolysis apparatus also features high quality electrodes made of platinum. The platinum electrodes contribute to the overall accuracy of readings and the durability of the equipment. However, because platinum electrodes cannot be plated, the Hoffman electrolysis apparatus is not able to weigh electric current. An electricity meter is needed to perform that function.
Accuracy is of utmost importance for an electrolysis apparatus, and the Hoffman electrolysis apparatus is designed to make it very easy for users to get accurate readings on a consistent basis. The Hoffman electrolysis apparatus collects hydrogen and oxygen separately, so the required two-to-one ration is easily maintained with a high degree of accuracy. Some models of Hoffman electrolysis apparatus are evn outfitted with pH indicators.
When using the Hoffman electrolysis apparatus, you will be able to tell whether or not gas is present in the tube quite easily. The presence of gas is indicated by a smoldering patch. When oxygen is present, you will see a vigorously burning white flame that burns very brightly. In cases where hydrogen gas is present, you will not see burning. Instead, the match will just glow. When current runs through your Hoffman electrolysis apparatus, the oxygen and hydrogen gases will cause displacement of water. The water will collect at the top of the tubes, and can then be drawn off for study.
While the ease of exacting measurement is a primary benefit of using the Hoffman electrolysis apparatus, this tool is also beneficial for qualitative study of the decomposition that occurs through electrolysis as well.
You can also find more info on Cost Of Electrolysis and Electrolysis Hair Removal. Electrolysisguide.com is a comprehensive resource to know about Electrolysis.
Source: www.articlecity.com